

Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys. Metallurgy encompasses both the science and the technology of metals; that is, the way in which science is applied to the production of metals, and the engineering of metal components used in products for both consumers and manufacturers. Metallurgy is distinct from the craft of metalworking.
Emerging areas for metallurgists include nanotechnology, superconductors, composites, biomedical materials, electronic materials (semiconductors) and surface engineering. Many applications, practices, and devices associated or involved in metallurgy were established in ancient China, such as the innovation of the blast furnace, cast iron, hydraulic-powered trip hammers, and double acting piston bellows. Read more
Strange Metal From Beyond Our Planet Spotted in Ancient Treasure Stash Science Alert - January 23, 2026

Australia's Buried Treasure: Rare Rocks Reveal Origins of a Critical Metal SciTech Daily - December 28, 2025

Niobium a transition metal that plays a critical role in modern materials science due to its ability to significantly enhance the strength, toughness, and corrosion resistance of alloys.

Ancient Writers Spoke Of A Mysterious Metal Called Orichalcum Linked To City Of Atlantis IFL Science - January 8, 2025
Orichalcum’s name is derived from the Greek for "mountain copper.” One of its most prominent mentions comes in the legend of Atlantis by Plato, in which it is described as more precious than anything except gold. The dialogue, called Critias, explains how the mythical citadel of Atlantis was adorned with walls, pillars, and floors that were coated in orichalcum, endowing the building with a flash of red light.
Often said to possess a reddish hue, there were many hints that orichalcum might be a form of brass - an alloy of copper and zinc - although its precise identity wasn’t revealed until several breakthroughs in modern science and archaeology.
In 2014, a diver discovered 40 ingots of an alloy metal in the Mediterranean Sea off the coast of the ancient Greek town of Gela in modern-day Sicily. Further surveys by the local authorities in 2016 revealed another 47 ingots just 10 meters (~33 feet) away from the first discovery. It was evident the two caches of ingots were from the same shipwreck that sunk to the seabed around 2,500 years ago. The skinny metal bars were found to be a copper-zinc alloy, suggesting they were a bundle of ancient orichalcum. In a strict sense, the term orichalcum should be understood to refer not to a single alloy but to a class of alloys that contained copper and zinc as principal components.

Orichalcum
Wikipedia
Strange Metal From Beyond Our Planet Found in an Ancient Treasure Stash

The Treasure of Villena, as the cache of 66 mostly gold objects is known, was discovered more than 60 years ago in 1963 in what is now Alicante in Spain, and has since come to be regarded as one of the most important examples of Bronze Age gold-smithing in the Iberian Peninsula, and the whole of Europe. Amidst a cache of glittering golden treasures from the Iberian Bronze Age, a pair of corroded objects might be the most precious of all. A dull bracelet and a rusted hollow hemisphere decorated with gold are forged, researchers have found, not out of metal from beneath the ground, but with iron from meteorites that fell from the sky. The discovery suggests that metalworking technology and techniques were far more advanced than we thought in Iberia more than 3,000 years ago.
A Cracked Piece of Metal Healed Itself in Experiment That Stunned Scientists Science Alert - September 2, 2024

File this under 'That's not supposed to happen!'. In an experiment, scientists observed a metal healing itself. If this process can be fully understood and controlled, we could be at the start of a whole new era of engineering.
That article took me back to my Roswell file ... Debris found at the scene of the Roswell UFO crash allegedly possessed highly anomalous physical properties. The strange material resembled dull aluminum or lead foil. When crumpled it straightened back up leaving no creases or wrinkles, similar to a shape memory alloy.

Newly discovered 'strange metal' does not follow traditional rules - could lead to deep insights PhysOrg - January 13, 2022
But in recent years, researchers have turned their attention to a class of materials that do not seem to follow the traditional electrical rules. Understanding these so-called "strange metals" could provide fundamental insights into the quantum world, and potentially help scientists understand strange phenomena like high-temperature superconductivity.
Billions of quantum entangled electrons found in 'strange metal' PhysOrg - January 16, 2020
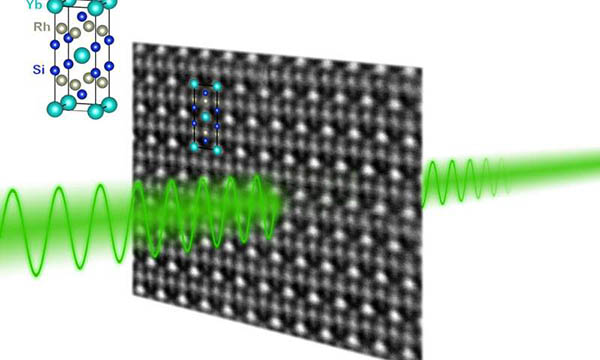
In a new study, U.S. and Austrian physicists have observed quantum entanglement among "billions of billions" of flowing electrons in a quantum critical material. The research examined the electronic and magnetic behavior of a "strange metal" compound of ytterbium, rhodium and silicon as it both neared and passed through a critical transition at the boundary between two well-studied quantum phases.