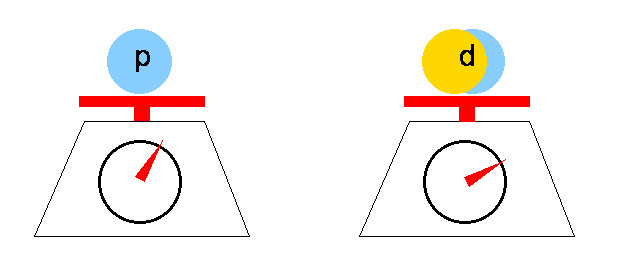

Heavy water is a form of water that contains a larger than normal amount of the hydrogen isotope deuterium (2 H or D, also known as heavy hydrogen), rather than the common hydrogen-1 isotope (1 H or H, also called protium) that makes up most of the hydrogen in normal water. The presence of deuterium gives the chemical different nuclear properties, and the increase of mass gives it different physical and chemical properties compared to normal "light water". Read more ...