

Chemistry (from Egyptian keme (chem), meaning "earth") is the science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions. It is a physical science for studies of various atoms, molecules, crystals and other aggregates of matter whether in isolation or combination, which incorporates the concepts of energy and entropy in relation to the spontaneity of chemical processes. Modern chemistry evolved out of alchemy following the chemical revolution (1773).

Disciplines within chemistry are traditionally grouped by the type of matter being studied or the kind of study. These include inorganic chemistry, the study of inorganic matter; organic chemistry, the study of organic matter; biochemistry, the study of substances found in biological organisms; physical chemistry, the energy related studies of chemical systems at macro, molecular and submolecular scales; analytical chemistry, the analysis of material samples to gain an understanding of their chemical composition and structure. Many more specialized disciplines have emerged in recent years, e.g. neurochemistry the chemical study of the nervous system.
Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds. Chemistry is sometimes called "the central science" because it connects physics with other natural sciences such as geology and biology Chemistry is a branch of physical science but distinct from physics. It is the science of atomic matter (that made of chemical elements), its properties, structure, composition and its changes during interactions and chemical reactions. Read more...
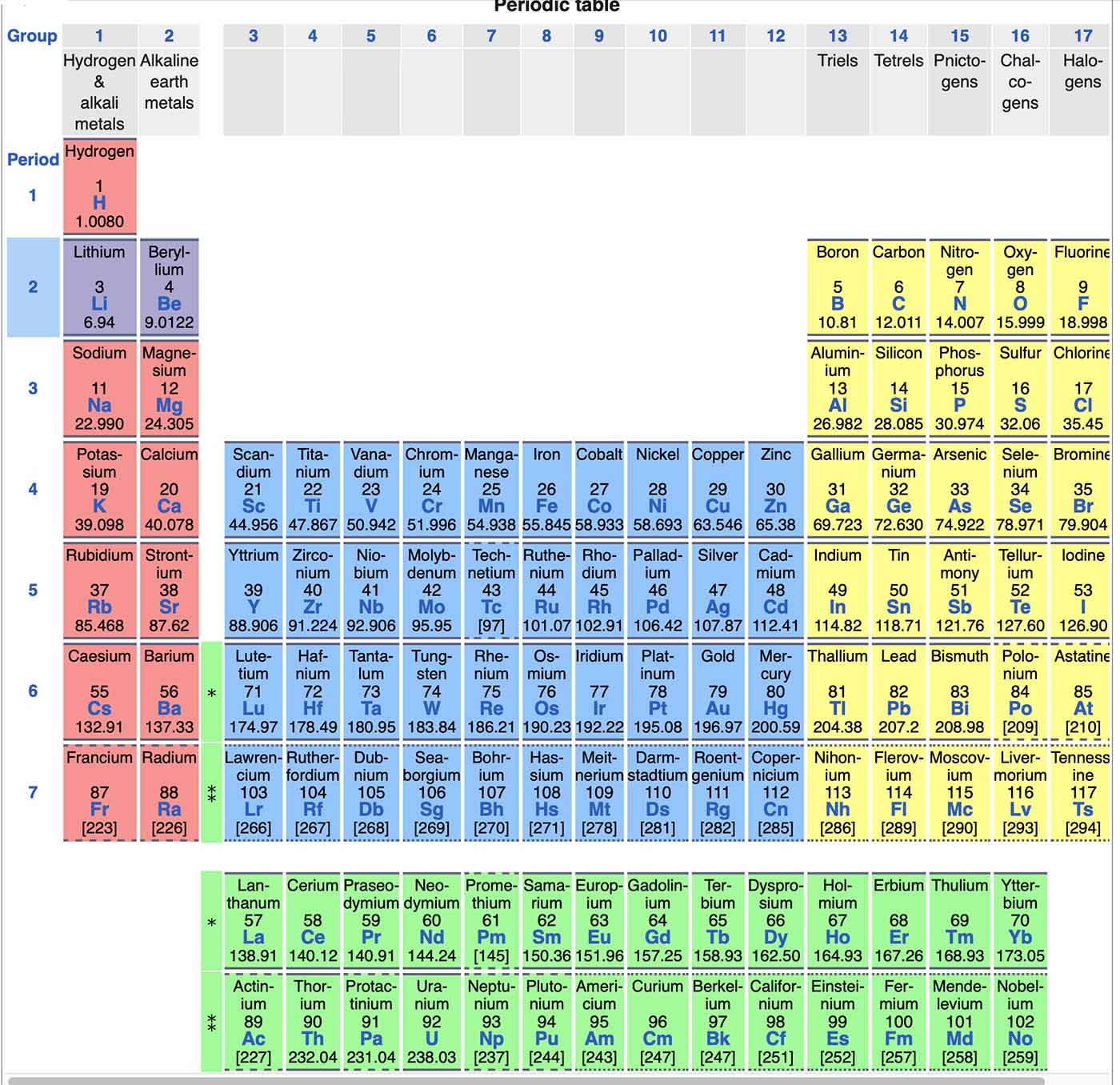
Periodic Table - Wikipedia
Scientists Unveil Eco-Friendly Breakthrough To Eliminate Forever Chemicals SciTech Daily - December 18, 2025

Researchers have created a new environmentally friendly method that can quickly capture and break down toxic forever chemicals (PFAS) in water. The results represent an important advance in efforts to address one of the most stubborn forms of environmental contamination.
Scientists Discover Highly Energetic Water Hiding in Plain Sight SciTech Daily - November 18, 2025
Water behaves differently when trapped in microscopic spaces instead of flowing freely. Researchers have shown that this confined water becomes highly energetic, influencing how molecules bind together.
Researchers decode the chemistry behind a deadly genetic disorder PhysOrg - November 18, 2025
Researchers used an original machine learning tool to predict how genetic mutations cause a rare metabolic disease known as OTC deficiency, uncovering some underlying biochemical mechanisms at play and laying the groundwork for future treatments
Molecules can only be produced in chemical reactions ... PhysOrg - November 17, 2025
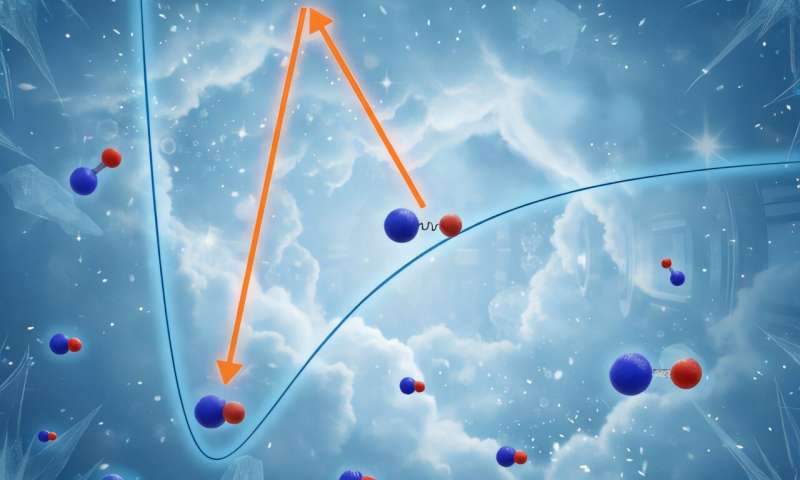
In the last 20 years, several different types of molecules have been produced in gaseous mixtures at temperatures close to absolute zero, using methods that narrow the exact time at which the molecules are made to a few microseconds.
On this date - Chemists discover buckyballs - the most perfect molecules in existence - Nov. 14, 1985 Live Science - November 14, 2025
Over a feverish 10-day period, scientists synthesized and described a new class of carbon molecules, called buckminster fullerenes, after the iconic 20th-century inventor.
From Plastic to Pure Water: Scientists Turn Trash Into a Super Catalyst
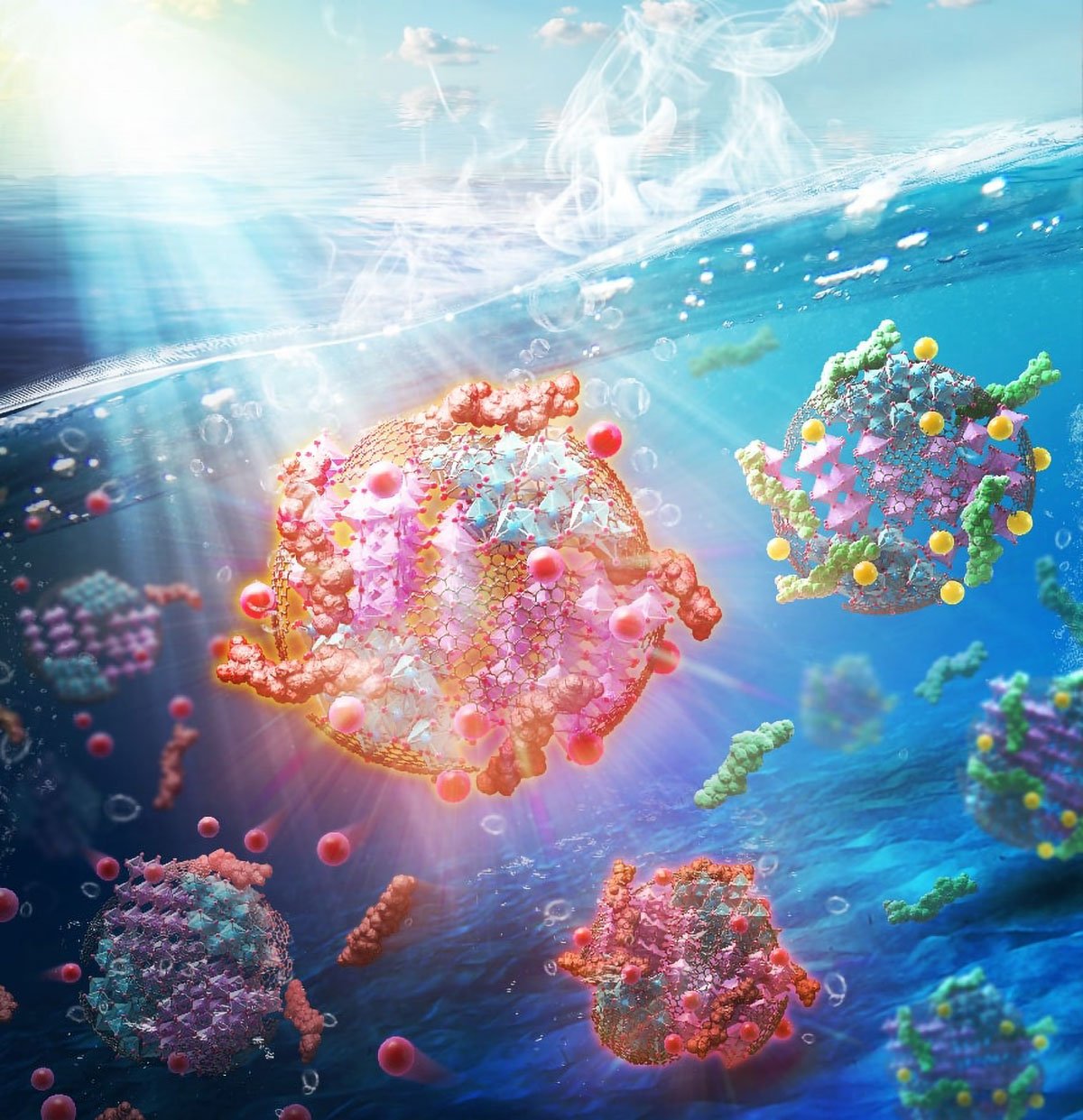
An optimized mechano-chemical process produces multifunctional composite particles that remove pollutants from water.
Scientists Develop More Efficient Way To Extract Rare Earth Elements Amid Global Trade Tensions SciTech Daily - November 13, 2025
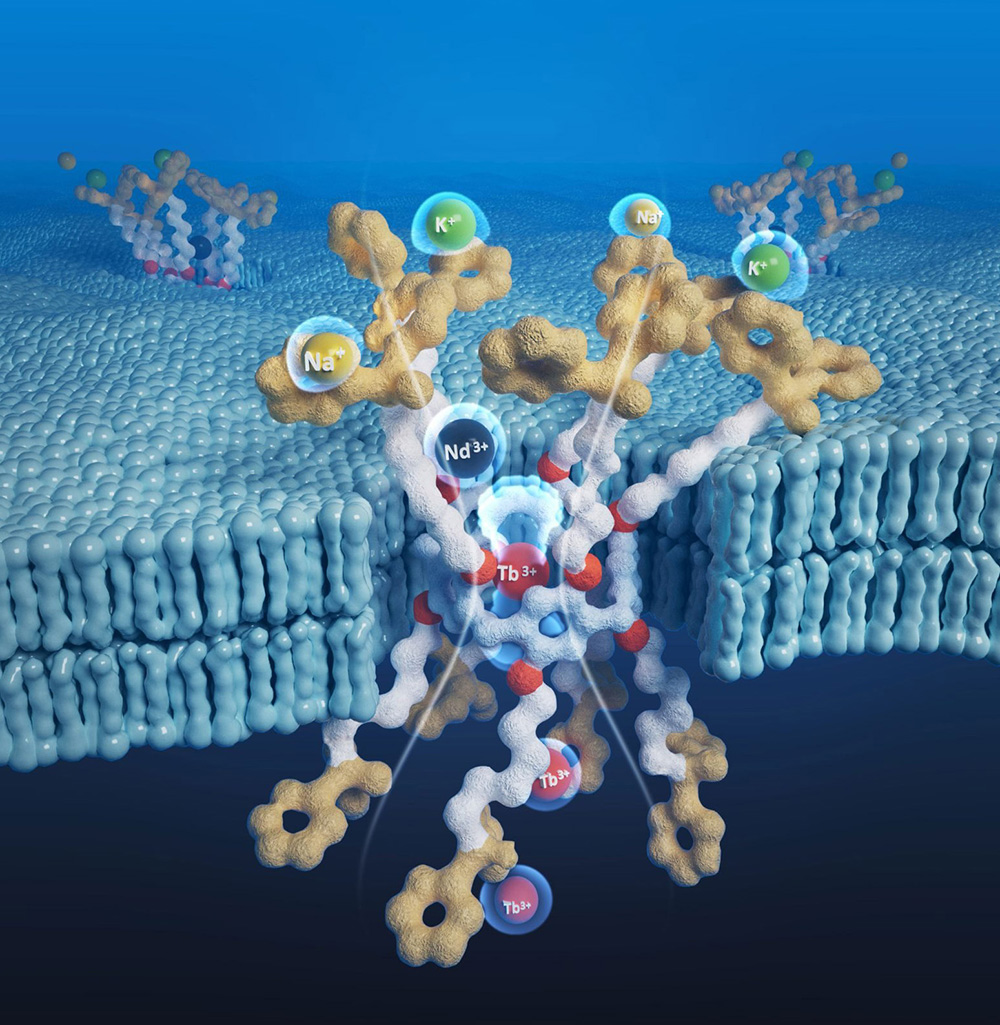
A team of scientists at The University of Texas at Austin has created a cleaner and more efficient way to extract rare earth elements, which are vital for technologies such as electric vehicle batteries and smartphones. The technique could strengthen domestic production and lessen dependence on expensive imports.
Scientists Solve the 2,000-Year-Old Mystery of Sea Silk's Unfading Gold SciTech Daily - November 10, 2025

Researchers Crack Decades-Old Chemistry Challenge SciTech Daily - November 3, 2025

A computational method accurately predicts the optimal ligand for a photochemical palladium catalyst, enabling new radical reactions of alkyl ketones.
Oldest Air Ever Measured Found in Ice From 6 Million Years Ago Excavated from deep under the surface of Antarctica Science Alert - November 1, 2025

From beneath hundreds of meters of glacial ice that gradually accumulated over eons at Allan Hills, a team of scientists led by glaciologist Sarah Shackleton of the Woods Hole Oceanographic Institute has retrieved samples that have been buried for some 6 million years.
-
In chemistry, air is a gas mixture that is primarily nitrogen (\(78\%\)) and oxygen (\(21\%\)), with small amounts of argon, carbon dioxide, water vapor, and other gases. Its chemical properties are understood through its components and physical laws like Dalton's law of partial pressures. Because it is a mixture, air does not have a single chemical formula, but its components can be separated by physical processes like fractional distillation of liquefied air.
Physicists unlock secrets of stellar alchemy, yielding new insights into gold's cosmic origins PhysOrg - October 24, 2025
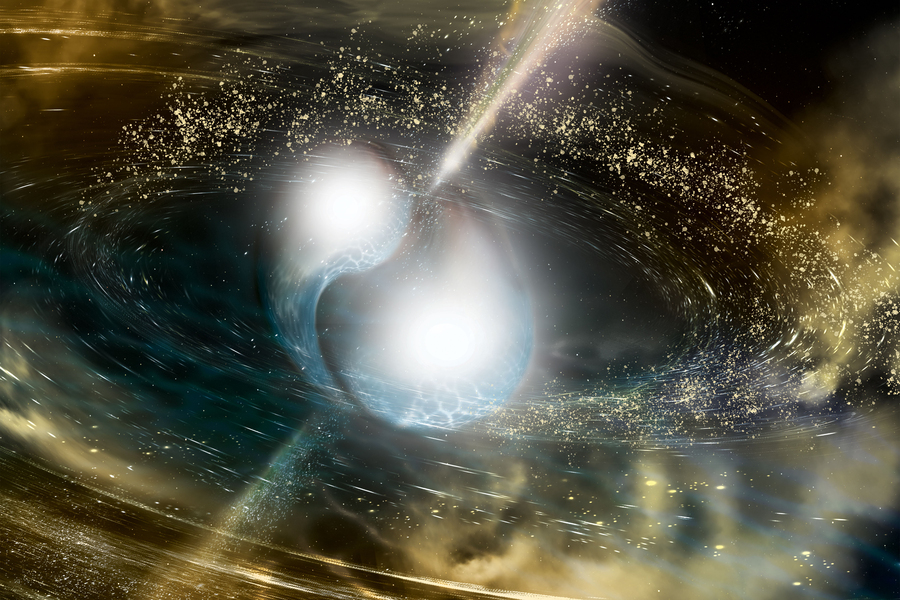
You can't have gold until a nucleus decays. The specifics of that process have been hard to pin down, but UT's nuclear physicists have published three discoveries in one paper explaining key details. The results can help scientists come up with new models to describe the stellar processes that give us heavy elements, as well as make better predictions about the expanding landscape of exotic nuclei.
Gold flakes expose the secret forces binding our world together Science Daily - October 24, 2025

Chalmers researchers have developed a simple, light-based platform to study the mysterious 'invisible glue' that binds materials at the nanoscale. Gold flakes floating in salt water reveal how quantum and electrostatic forces interact through vivid color changes. The technique could lead to new discoveries in physics, chemistry, and biology - from designing biosensors to understanding how galaxies form.
Bizarre Crystals in Titan's Lakes Could Break a Fundamental Rule of Chemistry Science Alert - October 23, 2025

A discovery about Saturn's moon Titan has challenged what scientists thought was a basic rule of chemistry.
Physicists unlock secrets of stellar alchemy, yielding new insights into gold's cosmic origins PhysOrg - October 22, 2025

Elements like gold and platinum are created under extreme conditions, such as when stars collapse, explode, or collide. In the rapid neutron-capture process (or r-process for short), a nucleus captures a barrage of neutrons in quick succession until it becomes so heavy it decays into lighter, more stable nuclei.
Nobel Prize in chemistry goes to scientist trio for Harry Potter-like work in molecular architecture CNN - October 8, 2025
A trio of scientists have been given the 2025 Nobel Prize in chemistry for the development of 'metal-organic frameworks,' a new form of molecular architecture likened to something from the Harry Potter novels. Susumu Kitagawa, Richard Robson and Omar Yaghi will share the prize for providing chemists with new opportunities for solving some of the challenges the field faces.
Splitting water: How order and disorder direct chemical reactivity PhysOrg - October 7, 2025
In nature, the behavior of systems - whether large or small - is always governed by a few fundamental principles. For instance, objects fall downward because it minimizes their energy. At the same time, order and disorder are key variables that also shape physical processes. System - especially our homes - tend to become increasingly disordered over time. Even at the microscopic level, systems tend to favor increased disorder, a phenomenon known as an increase in so-called entropy. These two variables - energy and entropy -play an important role in chemical processes. Processes occur automatically when energy can be reduced or entropy (disorder) increases.
Chemists Have Replicated a Critical Moment in The Creation of Life Science Alert - September 2, 2025

The spontaneous coalescence of the molecules that led to life on primordial Earth, some 4 billion years ago, may have finally been observed in a laboratory. Replicating the likely conditions of our newborn planet, chemists have joined together RNA and amino acids – the crucial first step that would eventually lead to the proliferation of living organisms that crawl all over Earth today. The experimental work could yield important clues about the origins of one of the most important biological relationships: the one between nucleic acids and proteins.
Earth's Core Could Be Hiding a Vast Reservoir of Primordial Helium Science Alert - March 4, 2025

The surprise discovery that one of the lightest elements in the Universe can bind to iron under high pressure to form iron helide means we may have misunderstood the chemistry making up the profoundest depths of our planet.
Scientists just got 1 step closer to creating a 'superheavy' element that is so big, it will add a new row to the periodic table Live Science - November 7, 2024
Scientists have discovered a new way of creating superheavy elements by firing supercharged ion beams at dense atoms. The team believes this method could potentially help synthesize the hypothetical "element 120," which would be heavier than any known element.
Scientists develop 'X-ray vision' technique to see inside crystals PhysOrg - June 4, 2024

A team of New York University researchers has created a new way to visualize crystals by peering inside their structures, akin to having X-ray vision. Their new technique which they aptly named "Crystal Clear" - combines the use of transparent particles and microscopes with lasers that allow scientists to see each unit that makes up the crystal and to create dynamic three-dimensional models.
Secrets of radioactive promethium - a rare earth element with mysterious applications - uncovered after 80-year search Live Science - May 29, 2024
For the first time, scientists have revealed crucial properties of the mysterious, radioactive substance promethium - nearly eight decades after the elusive rare earth element was discovered. Promethium is one of the 15 lanthanide elements at the bottom of the periodic table. Also known as the rare earths, these metals exhibit a number of useful properties, including strong magnetism and unusual optical characteristics, making them particularly important in modern electronic devices. They are used in lasers; they are part of the screens of your smartphone.
Strange Phase of Matter That Only Existed in Theory Turns Out to Be Real Science Alert - February 24, 2024

A strange phase of matter that previously existed purely in the realm of theory has finally been detected in a real material. It's known as the Bragg glass phase - a strange, seemingly paradoxical arrangement of atoms in a glass material where the particles are nearly as ordered as those in a perfect crystal. Scientists weren't even sure Bragg glass existed, but there it was, hiding in an alloy of palladium inserted between layers of terbium and tellurium (PdxErTe3).
Scientists Transformed Pure Water Into a Metal (Videos) Science Alert - February 7, 2024

By bringing pure water into contact with an electron-sharing alkali metal - in this case an alloy of sodium and potassium - free-moving charged particles can be added, turning water metallic.
For The First Time, Physicists 'Entangle' Individual Molecules With Staggering Precision Science Alert - December 14, 2023

Bulky and hard to wrangle, molecules have long defied physicists' attempts to lure them into a state of controlled quantum entanglement, whereby the molecules are intimately linked even at a distance. Now, for the first time, two separate teams have succeeded in entangling pairs of ultra-cold molecules using the same method: microscopically precise optical 'tweezer traps'.
Only 1% of chemicals in the universe have been discovered. Here's how scientists are hunting for the rest Live Science - October 18, 2023
The universe is flooded with billions of chemicals, each a tiny pinprick of potential. And we've only identified 1% of them. Scientists believe undiscovered chemical compounds could help remove greenhouse gases, or trigger a medical breakthrough much like penicillin did.
Ancient architecture inspires a new way to work with metal-organic frameworks PhysOrg - October 3, 2023
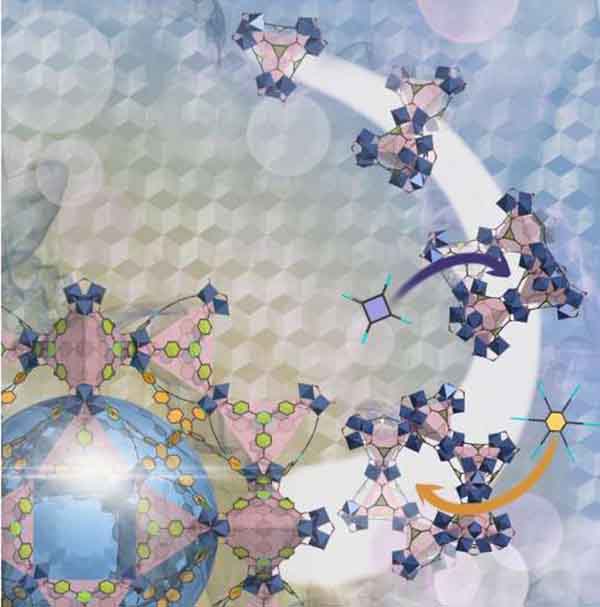
A centuries-old technique for constructing arched stone windows has inspired a new way to form tailored nanoscale windows in porous functional materials called metal-organic frameworks (MOFs). The method uses a molecular version of an architectural arch-forming "centering formwork" template to direct the formation of MOFs with pore windows of predetermined shape and size. New MOFs designed and made in this way range from narrow-windowed materials with gas separation potential to larger-windowed structures with potential medical applications due to their excellent oxygen-adsorption capacity.
Scientists theorize a hidden phase transition between liquid and a solid PhysOrg - August 15, 2023
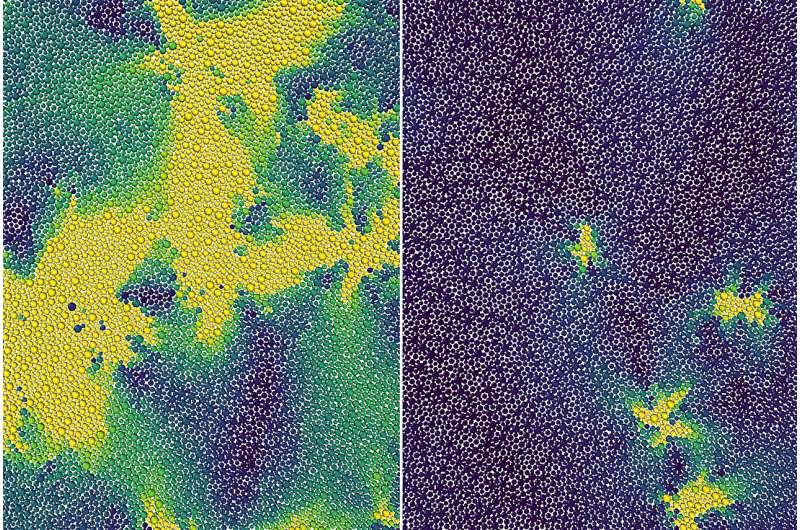
Researchers have discovered molecular behavior in supercooled liquids that represents a hidden phase transition between a liquid and a solid. Their improved understanding applies to ordinary materials like plastics and glass, and could help scientists develop new amorphous materials for use in medical devices, drug delivery, and additive manufacturing.
Hydrogen's Hidden Phase: Machine Learning Unlocks the Secrets of the Universe's Most Abundant Element Eureka Alert - April 23, 2023
Hydrogen under high temperatures and pressures will enhance our understanding of Jupiter and Saturn, gaseous planets primarily made of hydrogen. Hydrogen's simplicity makes the substance important to study.
RNA base in asteroid samples suggests origins of life on Earth. PhysOrg - March 25, 2023
Researchers found that approximately 50% of the Earth’s supply of the volatile element zinc came from asteroids originating from the outer Solar System, beyond the asteroid belt which encompasses planets such as Jupiter, Saturn, and Uranus. This material is believed to have also supplied other crucial volatiles, such as water
Scientists discover new 'origins of life' chemical reactions PhysOrg - July 28, 2022

Four billion years ago, the Earth looked very different than it does today, devoid of life and covered by a vast ocean. Over the course of millions of years, in that primordial soup, life emerged. Researchers have long theorized how molecules came together to spark this transition. Now, scientists at Scripps Research have discovered a new set of chemical reactions that use cyanide, ammonia and carbon dioxide - all thought to be common on the early earth - to generate amino acids and nucleic acids, the building blocks of proteins and DNA. They've come up with a new paradigm to explain this shift from prebiotic to biotic chemistry.
New Set of Chemical Reactions Could Finally Explain How Life Started on Earth Live Science - July 28, 2022
Once upon a time, when our planet Earth was very young and very new, there was not a single scrap of life on it to be found. Then, somewhere, somehow, some quirk of chemistry happened, and the molecular building blocks of our very first single-celled ancestors emerged: the amino acids and nucleic acids that came together in just the right way to continue a chain reaction that gave rise to life. We're not entirely sure of the details of this emergence, which took place billions of years ago, and left no trace on the fossil record. But using what we know of the chemistry of early Earth, scientists have found a new series of chemical reactions that could have produced those biological building blocks on Earth, all those eons ago.
Ultrahot 'superionic' ice is a new state of matter Live Science - November 15, 2021
Scientists just squeezed a water droplet between two diamonds and blasted it to star-like temperatures with one of the world's most powerful lasers. The result was a new and mysterious phase of water. Called superionic ice, the "strange, black" water exists under the same pressures and temperatures as those at the center of Earth - a fact that could soon help researchers investigate the secrets buried inside the cores of other worlds. Previously, researchers used shock waves to create this weird ice for just 20 nanoseconds before it dissolved. This new experiment marks the first time that scientists have created stable superionic ice that lasts long enough to be studied in detail.
Rare 'Alien' Isotopes in Earth's Crust Point to Recent Brush With a Cataclysmic Event Science Alert - May 15, 2021
Far down in the periodic table you'll find a list of heavy elements born in chaos. The kind of chaos you might find in an exploding star perhaps, or a collision between two neutron stars. Physicists have uncovered a pair of large, still-radioactive isotopes in samples of deep-sea crust pulled up from 1,500 meters (nearly 5,000 feet) below the Pacific Ocean. We'd expect to see many heavyweight elements in the swirl of dust and gas that formed our planet eons ago - but most should have decayed into more stable forms long before now. So finding examples in Earth's crust close to the surface today raises some interesting questions. The finding could tell us a thing or two about cataclysmic cosmic events taking place within a few hundred light-years from Earth, and relatively recently in our geological history. It could also shine a light on the way atomic heavyweights form.
Extraterrestrial Plutonium Atoms Turn Up on Ocean Bottom - The rare form of the element found on the Pacific seabed points to its violent birth in colliding stars Live Science - May 13, 2021
The plutonium-244 hints at how heavy metals form in stars. A rare version of the radioactive element plutonium embedded in Earth's crust below the deep sea is providing new clues as to how heavy metals form in the stars. The new research finds that the isotope, called plutonium-244, may arrive on Earth in tandem with iron-60, a lighter metal known to form in supernovas, explosions that occur during the death throes of many types of stars. This finding suggests that supernovas may create both heavy metals - although it's possible that other events, such as the mergers of neutron stars, are responsible for at least some of the plutonium-244. Understanding how heavy elements formed is one of the top three most burning questions in physics. Half of elements heavier than iron are built in the hearts of stars through a fairly well-understood process of fusion. The other half, though, requires a high density of free neutrons to form. This means they must form in a more explosive environment than a typical star core - supernovas, perhaps, or massive events such as a neutron-star merger or a collision of a black hole and a neutron star.
Mysterious Element 'Einsteinium' Measured by Scientists For The First Time Science Alert - February 3, 2021
For the better part of 70 years, isotopes of einsteinium have proven frustratingly difficult to study. Either they're way too hard to make, or they have a half-life of less than a year, and what precious little is created begins to fall apart like a sandcastle at high tide. The element's behavior is presumed to follow the patterns of its less robust peers in the actinide series. That much is clear. But due to its sheer size, strange relativistic effects make it harder to predict how it will react in certain chemical processes.
Discoveries at the edge of the periodic table: First ever measurements of einsteinium PhysOrg - February 3, 2021
Chemists create and capture einsteinium, the elusive 99th element Live Science - February 3, 2021
Physicists Found a Brand-New Kind of Magnet Hiding in a Uranium Compound Live Science - February 9, 2019
Scientists have discovered a brand-new kind of magnet hiding out in a uranium compound. The compound, USb2 (a compound of uranium and antimony), a so-called "singlet-based" magnet, is novel in that it generates magnetism in an entirely different way than any other magnet known to scientists. Electrons, which are negatively charged particles, generate their own tiny magnetic fields. These fields have a "north" and "south" pole, a consequence of a quantum mechanical property known as spin. In most objects, these magnetic fields point in random directions, canceling each other out. (This is why your body isn't a giant magnet.) But in certain materials, those fields become aligned. When that happens, they create a magnetic field powerful enough to, for example, move a bunch of iron filing around or cause a compass to point north.
Europe's 'New' Periodic Table Predicts Which Elements Will Disappear in the Next 100 Years Live Science - January 25, 2019
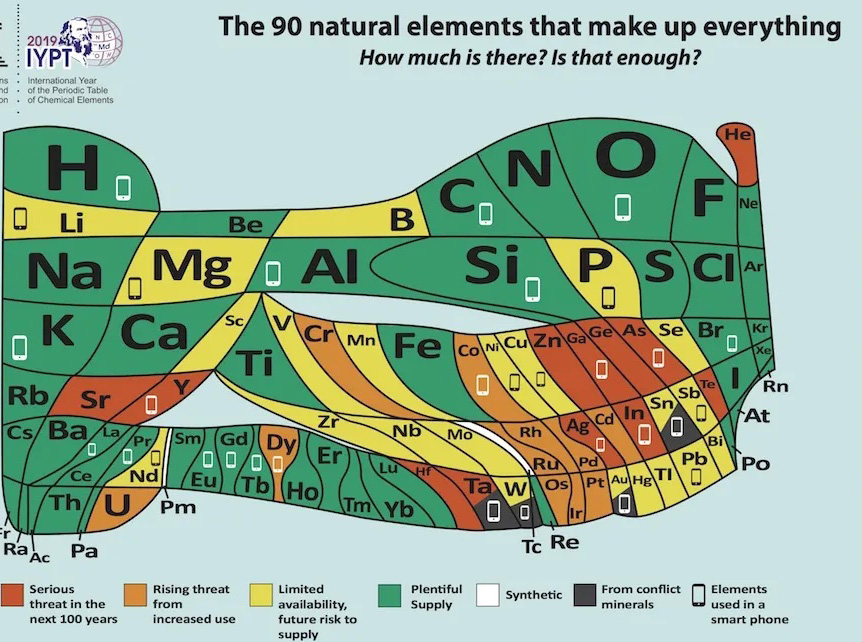
Oxygen can breathe easy, but the party might soon be over for helium balloons. Those are two takeaways from a brand-new model of the periodic table of elements, debuted this week by the European Chemical Society a group representing more than 160,000 chemists in the European Union. Unlike the ubiquitous classroom version of the table, which categorizes the universe's 118 known natural and synthetic elements with equal space for each element, EuChemS' chart has been warped and wobbled to show the relative abundance or scarcity of 90 naturally occurring elements here on Earth.
What a Tiny Electron Reveals About the Structure of the Universe Live Science - January 2, 2019
What is the shape of an electron? If you recall pictures from your high school science books, the answer seems quite clear: an electron is a small ball of negative charge that is smaller than an atom. This, however, is quite far from the truth. The electron is commonly known as one of the main components of atoms making up the world around us. It is the electrons surrounding the nucleus of every atom that determine how chemical reactions proceed. Their uses in industry are abundant: from electronics and welding to imaging and advanced particle accelerators. Recently, however, a physics experiment called Advanced Cold Molecule Electron EDM (ACME) put an electron on the center stage of scientific inquiry. The question that the ACME collaboration tried to address was deceptively simple: What is the shape of an electron?
As far as physicists currently know, electrons have no internal structure - and thus no shape in the classical meaning of this word. In the modern language of particle physics, which tackles the behavior of objects smaller than an atomic nucleus, the fundamental blocks of matter are continuous fluid-like substances known as "quantum fields" that permeate the whole space around us. In this language, an electron is perceived as a quantum, or a particle, of the "electron field." Knowing this, does it even make sense to talk about an electron's shape if we cannot see it directly in a microscope - or any other optical device for that matter?
>