
This is what a cat sees - Fuzzy but recognizable

| Research with Animals 1999 |
November 30, 1999 - AP - Springfield, Mo.
In an important milestone for an endangered species, an Asian elephant has given birth through artificial insemination.
Weighing in at 378 pounds, Haji was born Sunday at Dickerson Park Zoo. It was the world's first birth through artificial insemination of an Asian elephant, which are estimated to number only35,000 worldwide.
"I think this opens the possibilities in a number of areas, the main goal being the conservation of the animal," said Michael Hutchins, director of conservation and science for the American Zoo and Aquarium Association in Maryland."This is another way that captive animals can help preserve wild elephants and their place in nature," he said.
Veterinarians had tried unsuccessfully since the mid-1980s to impregnate an Asian elephant through artificial insemination. One problem is determining when the females breed in captivity. Another was the male's semen peaking early.
"We had to develop a technique to gather the semen and preserve its ability to fertilize," said Dickerson veterinarian Dennis Schmitt. "This all took a very long time."
Because male elephants are very aggressive, they need special holding pens and can't be kept with females, making breeding options difficult. Scientists hope artificial insemination will alleviate some of the expense and difficulty of transporting males between breeding facilities.
Meanwhile, Haji and mom Moola are bonding nicely. After 674 days in his mother's womb, Haji was standing with assistance 20 minutes later and nursing three hours later. The elephants will remain off exhibit for severalweeks. Zookeepers must now introduce Haji to the rest of the eight-elephant herd.


October 13, 1999 - BBC Online
These are the first pictures from an extraordinary experiment which has probed what it is like to look through the eyes of another creature.
As reported on BBC News Online last week, a team of US scientists have wired a computer to a cat's brain and created videos of what the animal was seeing.
By recording the electrical activity of nerve cells in the thalamus, a region of the brain that receives signals from the eyes, researchers from the University of California at Berkeley were able to view these shapes.
The team used what they describe as a "linear decoding technique" to convert the signals from the stimulated cells into visual images.
Dr Yang Dan, Assistant Professor of Neurobiology at UC Berkeley, Fei Li and Garrett Stanley, now Assistant Professor of Biomedical Engineering at Harvard University conducted 11 experiments.
They recorded the output from 177 brain cells that responded to light and dark in the cat's field of view.
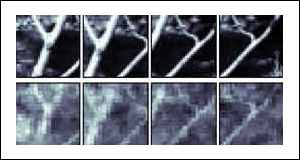
In total, the 177 cells were sensitive to a field of view of 6.4 by 6.4 degrees. As the brain cells were stimulated, an image of what the cat saw was reconstructed.
The first example is a face. Although the reconstructed image is rather fuzzy, it is clearly recognisable as a version of the original scene. It is possible that a clearer image could be obtained by sampling the electrical output of more cells.
In the cat's brain, as in ours, the signals from the thalamus cells undergo considerable signal processing in the higher regions of the brain that improve the quality of the image that is perceived.
Taking an image from a region of the brain before this image enhancement has taken place will result in a poorer image than the cat is able to see.
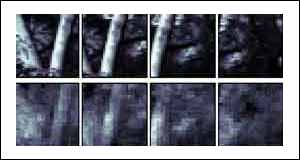
The other two examples show two woodland scenes, with tree trunks being the most prominent objects.
By being able to tap directly into the brain and extract a visual image the researchers have produced a "brain interface" that may one day allow the control of artificial organs and indeed machines by thought alone. It is also conceivable that, given time, it will be possible to record what one person sees and "play it back" to someone else either as it is happening or at a later date.
Frogs kiss and tell

September 30 1999 - Nature
Australian scientists have discovered the secret of attraction in frogs.
They have identified a pheromone - a powerful chemical signal - given out by male tree frogs during the breeding season to attract females.
The researchers, writing in the journal Nature, believe it is the first sex pheremone to be identified in frogs. It follows closely on research published two weeks ago which identified the chemical signal a salamander uses to woo his mate. That was thought to be the first identification of a vertebrate pheromone which affects female receptivity.
In this new work, Professor John Bowie and colleagues, of the University of Adelaide, South Australia, studied the male magnificent tree frog, Litoria splendida.
They discovered that males have glands in their heads from which they are able to squirt a molecule into water where the creatures breed.
Splendid chemical
This water soluble molecule, which the researchers have named splendipherin, instantly attracts female L. splendida frogs - even in tiny amounts.
The team tested it out by putting pads soaked in the perfume into special water tanks. When they put a female frog in the tank, she instantly homed in on the chemical from as far away as a metre and went and sat on the pad.
Just 40 nanograms (billionths of a gram) of splendipherin was sufficient to get a response. Smaller quantities of the pheromone got no reaction and larger ones seemed to confuse the animals.
Pheromones are now a popular subject for research. They are thought to play important roles in species recognition, reproduction and other behaviours.
Quite a few pheromones have been identified for insects, but only now are scientists beginning to describe these signalling molecules in vertebrate animal species.
Even in humans, pheromones are at work. Research has shown that the females of our species who spend a great deal of time in one another's company often send out chemical signals that help them synchronise their menstrual cycles.
The Adelaide team has already identified a molecule from L.

October 12, 1999 - Reuters
Somewhere in the Congo, a group of gorillas stands a better chance of survival because of DNA testing done far away at New York's Bronx Zoo.
Why would DNA testing help the gorillas survive?
The answer is that with DNA testing, scientists can determine breeding patterns. If they have enough DNA from enough different individuals, they can say who is related to whom and how closely each individual is related to every other individual.
This is important because inbreeding is a major reason that small populations of animals may die out. Inbreeding causes many problems; the more inbred a population is, the less resilience it has to environmental threats such as disease.
In the case of the Congo gorillas, local officials suspected that inbreeding might be a problem. The reserve that the gorillas inhabit is shaped like a giant hourglass, and officials there were concerned that the gorillas from one end of the reserve might not be crossing through the narrow part of the hourglass to reach mates on the other end.
If the two populations on either end of the hourglass were isolated from each other, the danger of inbreeding would be severe. But were they isolated? Or did they cross the narrow part of the reserve and mate with each other? Gorillas are shy and elusive creatures, and following them to determine their mating patterns would have been just about impossible. Nobody had a way of telling whether the gorillas were moving between the two sides of the reserve.
Nobody had a way, that is, until George Amato, a wildlife conservation specialist at the Bronx Zoo, and his colleagues reasoned that DNA testing could provide the answer. But how to get DNA samples if the scientists stood little chance of getting near even one of the gorillas, let alone all of them? The answer was that if they could get hairs shed from the gorillas' coats, the hairs would have all the DNA needed for the tests. Fortunately, there's a way to get hairs even when you can't get near the gorillas.
When a troop of gorillas stops for the night, each adult member of the troop will make a bed for itself from branches and leaves. Often a few hairs will stick to the gorillas' night nests.
Amato's colleagues searched for night nests, collected sample hairs and then sent them to Amato's laboratory at the zoo. There, Amato and his colleagues used DNA testing on the hairs, calculated the degree of inbreeding, and came to an alarming conclusion.
There was very little mating between the gorillas from the two sides of the reserve. The bottleneck formed by the shape of the reserve was keeping the two populations apart.
The DNA testing demonstrated that the reserve needed to be changed if the gorillas were to survive. Because of the Bronx Zoo tests, authorities are working to secure additional land at the bottleneck part of the reserve.
The gorillas would then feel more secure passing from one side of the reserve to the other, and there would be less inbreeding. One of the Congo's great natural treasures would then have a vastly improved chance of survival - thanks to DNA testing from the Bronx Zoo.
September 25, 1999 - Drudge report
Scientists have successfully incubated human eggs inside laboratory mice in research that raises fresh ethical concerns about the direction of reproductive medicine.
Two teams of researchers have independently shown it is possible to take immature egg cells from women's ovaries and grow them to full maturity inside a mouse. They believe the development offers a way of maturing the eggs of young women or girls who have had their ovaries removed before undergoing cancer treatment that would have left them permanently sterile.
The research will be greeted with dismay by organisations opposed to developments in human reproductive medicine or to animal experiments. But the breakthrough could benefit scores of women undergoing cancer therapy who have had their ovary tissue frozen in the hope that it might one day be used to help them have babies.
This week the world learned that a British scientist, Roger Gosden, professor of reproductive biology at Leeds University, had helped to pioneer the first ovary transplant, which promises to revolutionise female fertility by allowing the use of stored ovaries. Professor Gosden has also managed to take immature human eggs cells from an ovary and "ripen" them inside the body of an immune-deficient mouse, which does not reject "foreign" tissue.
The research was repeated by a team of reproductive scientists from the Toronto Hospital in Canada, who hope to use the technique to restore the fertility of cancer patients.
Professor Gosden said in his recent book, Designer Babies, that a trans-species "xenograft" should be used only in extreme circumstances. "If we did use them the animal would not, of course, become pregnant, as the egg would have to be removed and fertilised with a human sperm in vitro and then transferred to a human womb to stand any chance of pregnancy," he wrote. "I would not recommend growing eggs in animals until a great deal of research has been done to reassure us that there are no risks of viruses or prions [the proteins that cause BSE] jumping species and infecting the egg."
The Leeds team inserted the human ovarian follicles under the kidney membrane of the laboratory mouse where they grew to full maturity and were ready for transplantation into a human womb. The Toronto team grew the follicles under a mouse's skin. Half of the eggs matured.
Adam Balen, consultant obstetrician and gynaecologist at Leeds University, who worked with Professor Gosden, said the ethical and practical difficulties of using animals as living incubators makes the research less attractive than chemical cultivation of human eggs in the laboratory.

These piglets have been modified for transplants
Research led by British scientists has suggested that pig organs are safe to use in human transplants.
The study carried out in Cambridge found that people who receive transplanted pig organs are unlikely to contract incurable, infectious diseases.
There has been a widespread fear that if such operations were approved, pig viruses might spread to humans in a similar way that HIV seems to have done from chimps.
The study of 160 patients, who have been treated with various living pig tissues over the past 12 years, appear to show these fears are groundless.
The severe shortage of human transplant donors makes the use of organs from other species (xenotransplantation) an appealing alternative to many patients and doctors.
Pigs look to be the most promising source of these organs because the animals are close in size to humans and are easy to breed in large numbers.
Scientists have already made great progress in altering the genetic make-up of pigs so their tissues are less likely to be rejected by the human immune system.
The new data, published in the journal Science, will now intensify the pressure on regulatory authorities in a number of countries to allow full-organ transplant trials to proceed.
In Britain, the UK Xenotransplantation Interim Regulatory Authority (UKXIRA) will screen applications, with government ministers giving the final approval.
Health Secretary Frank Dobson has already said that safety is the key issue, having accepted that there should be no ethical objection to this kind of operation.
One of the frontrunners in this field is the UK biotechnology company Immutran Ltd, based in Cambridge. It was also responsible for leading this latest study on the dangers of pig viruses.
Of particular concern is the porcine endogenous retrovirus (Perv), which has become a permanent part of pigs' DNA and is passed on to new generations in eggs and sperm.
The authors looked at individuals who have already received pig tissue for a number of conditions for any signs that Perv had infected their cells. These patients included people who have received skin grafts, and diabetics who have been given insulin-making cells taken from the pancreas of pigs.
The study found no evidence of Perv infection, even in 36 patients who had their immune systems suppressed with drugs as part of their treatment and were therefore presumed to be at increased risk of infection.
Samples from some of the patients did contain some Perv DNA, but this appears to be due to the fact that these patients were still carrying pig cells in their bloodstream, a phenomenon known as "microchimerism".
The authors were surprised to learn that pig cells can last for so long in the patient - even 8.5 years after a transplant - but argue this merely shows that pig tissue can survive in the human body for long periods with no ill effects.
"We have learned a great deal from this study," said the lead author Dr Kaz Paradis, Director of Clinical Research at Imutran. "But it is important to remember that any move to clinical trials will only take place following open discussion with scientific and clinical experts, and with full approval from the appropriate regulatory authorities."
Professor Sir Roy Calne, a transplant pioneer who developed the anti-rejection drug cyclosporine A said: "This study adds considerably to the breadth and depth of the knowledge that the scientific community continues to accumulate as we explore the potential for xenotransplantation in the clinical setting."

However, Robin Weiss of the Institute of Cancer Research in London, issued a strong note of caution. It was his research that helped to provoke much of the recent concern after he demonstrated that two pig viruses could transfer to human cells under certain laboratory conditions.
Having reviewed the new data, he said the study "offered some reassurance" on safety. But he said "the ethical and technical problems of maintaining vigilance over xenotransplantation should not be underestimated."
"The transplant of pig tissue cannot be compared to transplanting a whole organ, which may be a larger reservoir of viruses," a spokeswoman said. "Also, negative results cannot be a complete proof: They may indicate an absence of viruses, but they may also merely reflect a failure to detect them."

'Pig hearts soon, cloned hearts later'
August 20, 1999 - BBC
One of the marvels of modern medicine is the organ transplant. Many people who would have died simply because their blood-pump stopped working properly are now alive and thriving because of heart transplants.
But there is a severe shortage of organs and many die before a suitable one becomes available.
The news that viruses found in pig tissue do not appear to infect humans should be welcomed because it overcomes one of the major practical barriers to using pig organs in humans.
Most people have no objection to using pigs - they are used for food already. We also use many tissues from pigs including heart valves and skin grafts already, so using an entire organ such as the heart to save a life should not raise any new ethical debate.
Pigs are favoured by scientists because they have many similarities to humans. Their hearts are about the same size as ours with similar plumbing and power output. What is more, they would require only a minor bit of genetic engineering to be compatible with our immune systems.
The scientists at the Roslin Institute who created Dolly are using this expertise to develop pig clones suitable for transplants. So expect the first pig hearts to be transplanted into humans in just a few years. But it may be a short-lived phase. The days when we crudely transplant a heart from a human or a pig may be numbered. The real breakthrough may come from cloning and tissue engineering research.
Why bother with adapting the cells and organs of other species when there is the possibility of manipulating human cells to do the same thing far more efficiently.
If you are unfortunate to have a diseased heart, what could be better than growing a new one out of your own cells? There would be no compatibility problems and surely no ethical ones.
This may be possible, or rather, will be possible in perhaps a decade. Across the world, many scientists are working to achieve this goal. A team from the University of Toronto has said it wants to create a tissue-engineered heart suitable for transplant within 10 years.
And a team of scientists from Boston, US, are developing a technique where you could grow a new organ actually inside your own body.
This will happen long before we clone a human being. We should not let ethical objections to cloning a human stop important research that would lead to such benefits.
ANIMAL RESEARCH
CLONING STEM CELL RESEARCH HEALTH INDEX ALPHABETICAL INDEX OF ALL FILES CRYSTALINKS MAIN PAGE